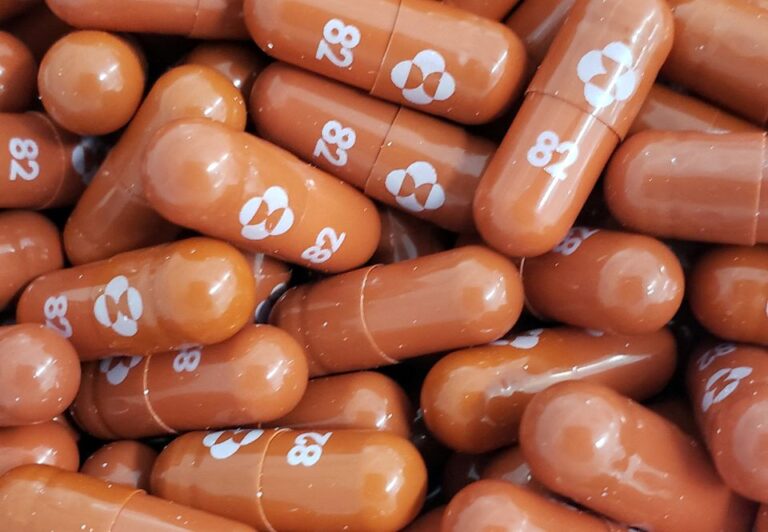
(Reuters) – An expert committee of India’s drug regulator recommended emergency use authorisation (EUA) for Merck’s (MRK.N) COVID-19 pill molnupiravir, and Serum Institute of India’s Covovax and Biological E’s corbevax vaccines, the Economic Times reported.
The recommendations by the subject expert committee have been sent to the Drugs Controller General of India, which will soon decide on their approval, according to the report on Tuesday.Data presented for Covovax, the Indian version of Novavax Inc’s (NVAX.O) COVID-19 vaccine, was satisfactory, the report said.
The Central Drugs Standard Control Organisation, India’s regulatory body for cosmetics, pharmaceuticals and medical devices, did not immediately respond to a Reuters request for comment.India is working on ramping up oxygen supplies and strengthening health infrastructure to contain a possible surge of COVID-19 cases due to the Omicron variant.The country has administered 1.43 billion COVID-19 vaccine doses so far, with more than 839 million of all adults having received at least one dose.
Last week, the U.S. FDA issued an EUA to molnupiravir for the treatment of mild-to-moderate coronavirus disease in adults.Earlier this year, drugmakers Aurobindo Pharma (ARBN.NS), Cipla (CIPL.NS), Sun Pharmaceuticals (SUN.NS) and some others signed non-exclusive voluntary licensing agreements with Merck to manufacture and supply molnupiravir in India.
The country plans to start administering COVID-19 booster shots as a precautionary measure to healthcare and frontline workers from Jan. 10 and will begin vaccinating those aged 15-18 from Jan. 3.Medical experts have said India needs to double down on its vaccine campaign and some states have imposed night curfews and other restriction as a precaution in the run up to New Year festivities to prevent a spike in infections and a repeat of summer 2021 when a devastating second wave of infections left tens of thousands dead.






